Research
Two-dimensional oxide quasicrystals
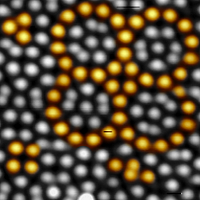
Scanning tunneling microscopy image showing the atomic structure of an oxide quasicrystal surface. The building blocks of the aperiodic tiling are emphasized. Neighboring atoms are found in 0.7 nm distances.
With the discovery of two-dimensional quasicrystals from BaTiO3 on Pt(111) in our group in 2013 a new class of materials has been added to the family of quasicrystals. Besides quasicrystals from metal alloys and organic materials, for the first time an oxide has been found to develop an aperiodic long-range order. These oxide quasicrystals are monoatomically thin layers spreading under reducing conditions across metal surfaces. They are characterized by a twelvefold rotational symmetry that comes along with a lack of translational symmetry in the long-range ordered film.
By utilizing scanning tunneling microscopy, the quasicrystal tiling has been imaged on the atomic scale and it turned out that the tiling consists of atomic arrangements in equilateral triangles, squares and rhombuses. A statistical analysis of the oxide quasicrystal tiling worked out that it is the first experimental observation of a dodecagonal tiling introduced by Niizeki and Gähler more than 30 years ago.
A junior research group headed by Dr. Stefan Förster systematically investigates the formation of oxide quasicrystals. So far oxide quasicrystals fromed from BaTiO3 SrTiO3 on Pt(111) surfaces are known. However, there is manifold of combinations of chemically related metal cations into two-dimensional ternary oxides, which also could lead to the formation of oxide quasicrystals. A broad spectrum of methods is applied to study these two-dimensional oxides including diffraction (LEED, SXRD), microscopy (STM, AFM, PEEM, LEEM), and spectroscopies (XPS, UPS, ARUPS, 2PPE, STS, HREELS).
An additional focus is put on investigations of the phason dynamics of oxide quasicrystals. Phasons are lattice excitations of the higher-dimensional periodic hyperspace of these quasicrystals. In the framework of a joint network of three french and three german institutions, which is co-funded by ANR and DFG, we conduct synchrotron-based temperature-dependent SXRD measurements and high-T STM measurements to tackle this topic.
Alkali-induced surface defects and interface passivation in
chalcogenide solar cells
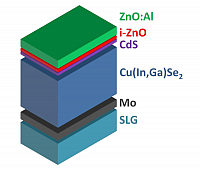
Schematic representation of a chalcogenide solar cell.
With the materials:
ZnO:Al - aluminium doped zinc oxide
i-ZnO - intrinsic zinc oxide
CdS - cadmium sulfide
Cu(In,Ga)Se2 - CIGS
Mo - molybdenum
SLG - soda-lime-glass
Solar cells are one of the most famous technologies for turning renewable energies into electricity. One part of scientific interest to the photovoltaics are thin-film solar cells. These are a combination of different material systems. CIGS (copper-indium-gallium-diselenide) is the conducting layer. By adding an alkali metal like sodium to CIGS it is possible to increase the solar cell efficiency.
The group of photovoltaics from Prof. R. Scheer showed that illumination of CIGS leads to a reduction of the solar cell efficiency if the CIGS surface is in contact with oxygen during the illumination. With the time-resolved photoluminescence (trPL) measurement one can reason about the behaviour of the solar cell efficiency.
The surface science group can measure the chemical composition of materials via x-ray photoelectron spectroscopy (XPS).
By combination of trPL- and XPS measurements it was shown that the reduction of the solar cell efficiency is due to an diffusion of sodium in combination with oxygen to the surface of CIGS (see paper: https://doi.org/10.1063/1.4992116 ).
The combination of trPL and XPS is pioneering and will help to figure out the role of alkali metals in chalcogenide thin-film solar cells.
Nanometer-resolved ultrafast spin and electron dynamics at magnetic surfaces
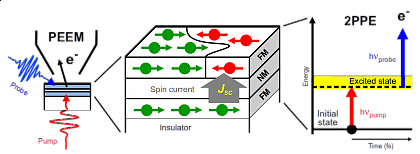
The goal of our joint CRC/TRR 227 with the FU Berlin is to establish a fundamental understanding of ultrafast spin dynamics, thereby laying the foundations for spin-based information technology. Therefore, our first goal is to gather and develop detailed knowledge about spin dynamics and the couplings of electron, phonon, and spin degrees of freedom on ultrafast time scales.
In this project the spin and magnetization dynamics of ferromagnetic and antiferromagnetic thin films are studied on the nanometer length scale. Femtosecond time-resolved photoemission electron microscopy (tr-PEEM) is applied in combination with time-resolved two-photon photoelectron spectroscopy (tr-2PPE). With independently tunable pump and probe laser pulse wavelengths, we address the spatial distribution of spin dynamics driven by a transient electronic structure. This transient electronic structure under investigation will be excited indirectly by a spin current or directly by optical pulses. These excitations will be investigated by tr-2PPE and spatially tailored by plasmonic nanostructures in the tr-PEEM experiments. A specific goal is to image domain switching and domain wall motion in ferromagnetic and antiferromagnetic thin films on the nanometer scale upon femtosecond spin and optical excitations.




